Research

Research Summary: Mechanism of response, toxicities and resistance to cancer immunotherapy
Our lab focuses upon defining the mechanisms that underlie responses, immune toxicities and resistance to cancer immunotherapy. We study approaches to prime and potentiate endogenous immune responses in tumor-bearing individuals. Our initial work was on autologous dendritic cell vaccines targeting shared tumor associated antigens, both in preclinical models and also in patients. This helped to lay the groundwork for the FDA-approved cancer vaccine sipuleucel-T. We have continued to study how approaches for endogenous vaccination with cancer therapies can serve to prime anti-tumor immune responses.
We are also studying the impact of targeting immune checkpoints and conventional cancer therapies. We use mouse models to dissect specific immune effectors (including T cells and B cells) in mediating responses to different immunotherapies. Using this approach, we have identified novel mechanisms of resistance to immunotherapy, which we have shown to be at work in cancer patients. With this information, we can develop treatment regimens to improve treatment response in patients.
We also examine these immune mechanisms and potential biomarkers in patients receiving immunotherapies. We are tracking antigen specific immune responses through the use of MHC-peptide tetramers and high-throughput sequencing of T cell antigen receptors (TCR-seq). We also utilize single cell techniques including high dimensional flow cytometry as well as next generation sequencing (single-cell RNA-seq) to determine the effects of immunotherapy on the functional states of immune cells. Future work will focus on identifying novel determinants of response as well as novel targets for future therapies.
Examples of Ongoing Research:
Clinical trials
Clinical trials that are both ongoing and completed can be found at ClinicalTrials.gov
Immune monitoring of clinical trials
Blood and tissues samples are collected with patients’ consent and de-identified following HIPAA regulations. All samples are processed for immediate ex vivo experiments or for storage in order to batch samples for future experiments in cryogenic conditions following standard operating procedures (SOP) to maintain consistency by highly trained staffs.
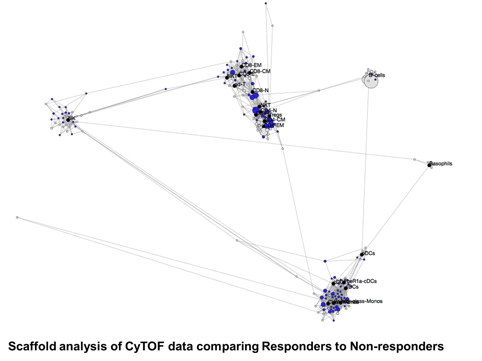
We are using CyTOF (Cytometry Time-Of-Flight) to carry out immune monitoring of patients’ samples before and after immunotherapy treatment when available. CyTOF (Fluidigm) is a variation of flow cytometry in which antibodies are labeled with heavy metal ion tags which are detected by time-of-flight mass spectrometry. This allows for the combination of many more antibody specificities in a single sample, without significant spillover between channels. Each panel has over 35 antibodies designed to look broadly or on specific cell types like T, myeloid and NK cells based on the type of immunotherapy treatment and the questions investigated.
The objectives are 1) to determine the effects of immunotherapy by comparing post and pre-treatment timepoints; 2) to determine if the timing of immunotherapy impacts the immunomodulation of activated effector and regulatory T cells; 3) to determine which pre-existing or treatment-induced immune responses may correlate with clinical outcomes.
Investigation of tumor-infiltrating lymphocytes in tumors of patients treated with checkpoint immune blockade compared to patients not receiving immunotherapy by single-cell RNA-seq and TCR-seq
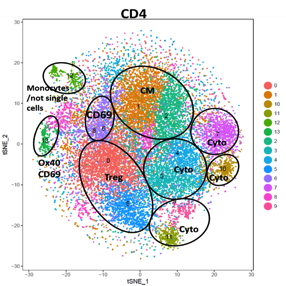
Certain cancers are responsive to immunotherapies such as PD-1 blockade, but rates of clinical response are low. While cytotoxic CD8+ T cells are thought to mediate tumor rejection, the contribution of other tumor-resident T cells, which may possess heterogeneity in their antigenic repertoire and function, is unknown. We are performing droplet single-cell RNA and paired T cell receptor sequencing of T cells isolated from localized tumors and paired adjacent non-malignant tissue from patients with localized cancer. We found that while CD8+ T cells are thought to be critical for tumor rejection, the composition and repertoire restriction of CD8+ populations are not altered in tumors. In contrast, effector populations of CD4+ T cells develop several distinct tumor-specific states including novel populations of cytotoxic CD4+ T cells, which are clonally expanded in tumor and can kill autologous tumor cells. Further studies are underway to characterize these populations and to determine if the same populations and cells with tumor-specific TCR can be similarly detected in the blood. The ability to detect and distinguish these cells in the blood will enable the ability to test them for their use as potential biomarkers for better patient selection.
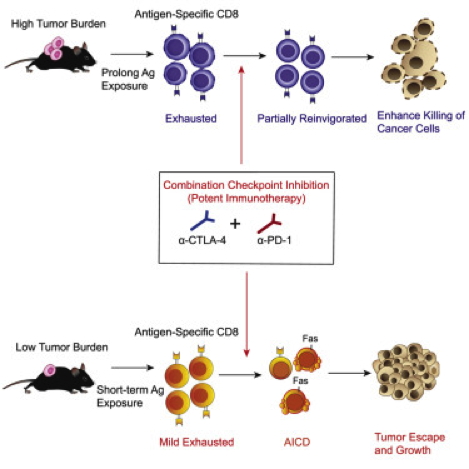
Mechanism of resistance to checkpoint-blockade treatment in mouse models with transplantable tumors
We found that although treatment with combined anti-CTLA-4 and anti-PD-1 improved control of established tumors, this combination compromised anti-tumor immunity in the low tumor burden (LTB) state in pre-clinical models as well as in melanoma patients. Activated tumor-specific T cells expressed higher amounts of interferon-γ (IFN-γ) receptor and were more susceptible to apoptosis than naive T cells. Combination treatment induced deletion of tumor-specific T cells and altered the T cell repertoire landscape, skewing the distribution of T cells toward lower-frequency clonotypes. Additionally, combination therapy induced higher IFN-γ production in the LTB state than in the high tumor burden (HTB) state on a per-cell basis, reflecting a less exhausted immune status in the LTB state. Thus, elevated IFN-γ secretion in the LTB state contributes to the development of an immune-intrinsic mechanism of resistance to combination checkpoint blockade, highlighting the importance of achieving the optimal magnitude of immune stimulation for successful combination immunotherapy strategies